CryoEM Structure of metazoan Mon1-Ccz1-RMC1 complex
Jia, G.W., Yong, X., Su, Z.M., Jia, D.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| MIP05619p | 642 | Drosophila | Mutation(s): 0 Gene Names: CG8270-RA | 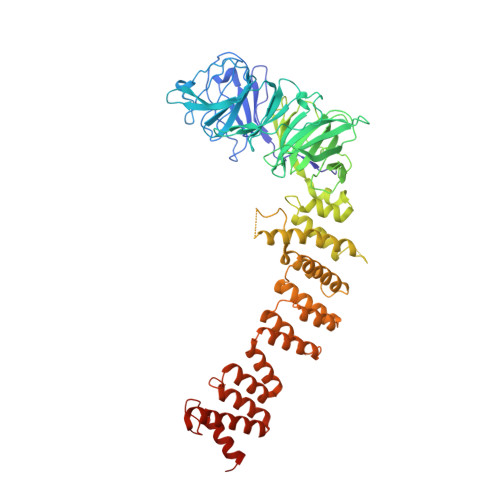 | |
UniProt | |||||
Find proteins for Q9VRX1 (Drosophila melanogaster) Explore Q9VRX1 Go to UniProtKB: Q9VRX1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9VRX1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Caffeine, calcium, zinc sensitivity 1 | 485 | Drosophila | Mutation(s): 0 Gene Names: Ccz1, C7orf28B, Dccz1, Dmel\CG14980, NP_647835, CG14980, Dmel_CG14980 | 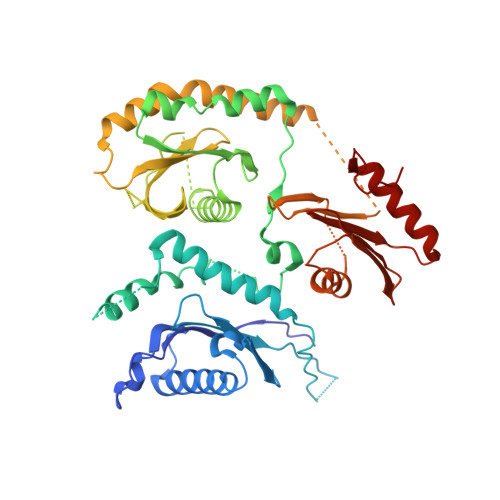 | |
UniProt | |||||
Find proteins for Q9VZL5 (Drosophila melanogaster) Explore Q9VZL5 Go to UniProtKB: Q9VZL5 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9VZL5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Vacuolar fusion protein MON1 homolog | 548 | Drosophila | Mutation(s): 0 Gene Names: Mon1, Dmel\CG11926, Dmon1, MON1, Mon1-RA, NP_608868, CG11926, Dmel_CG11926 | 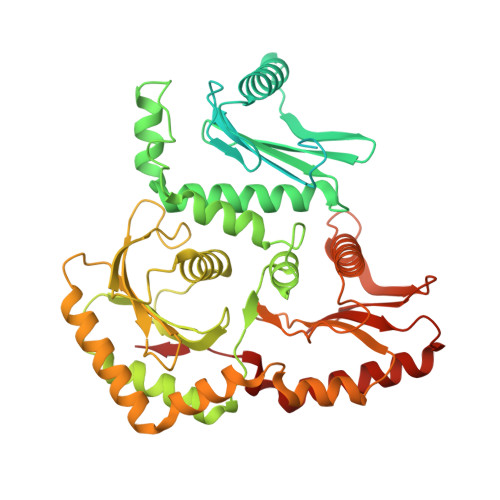 | |
UniProt | |||||
Find proteins for Q9VR38 (Drosophila melanogaster) Explore Q9VR38 Go to UniProtKB: Q9VR38 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9VR38 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 32222040 |
| National Natural Science Foundation of China (NSFC) | China | 32070049 |
| Ministry of Science and Technology (MoST, China) | China | 2022YFC2303700 |
| Ministry of Science and Technology (MoST, China) | China | 2021YFA1301900 |